

MOLECULAR MADE EASY
3-in-1, single-use, molecular testing for COVID-19, Flu A, and Flu B1
(FOR AGES 2 AND UP)
LUCIRA® by Pfizer COVID-19 & Flu Test is a single-use combined COVID-19, Flu A, and Flu B testing device for fast, accurate,* point-of-care testing powered by molecular technology.1
EMERGENCY USE AUTHORIZATION
The LUCIRA® by Pfizer COVID-19 & Flu Test has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection and differentiation of nucleic acid from SARS-CoV-2, influenza A, and influenza B, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Fast.
Determine if viral respiratory symptoms are due to COVID-19, Flu A, and/or Flu B in 30 minutes or less.1

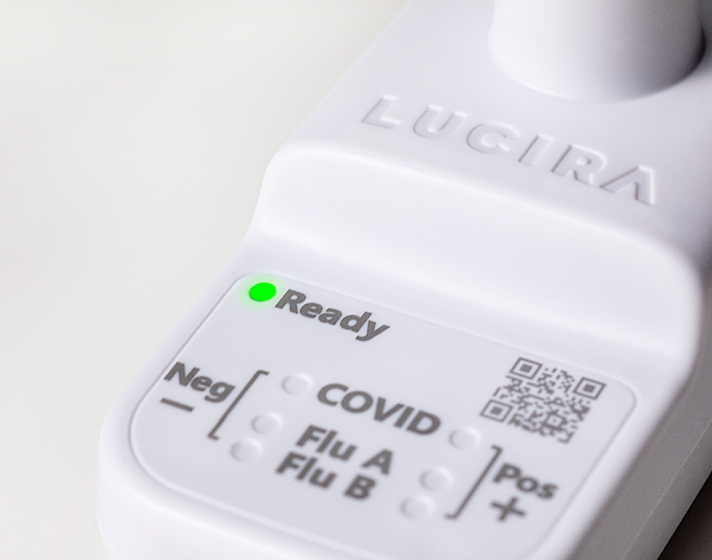
Accurate.
LUCIRA® by Pfizer uses molecular technology to deliver lab-quality accuracy* in a palm-sized device.1
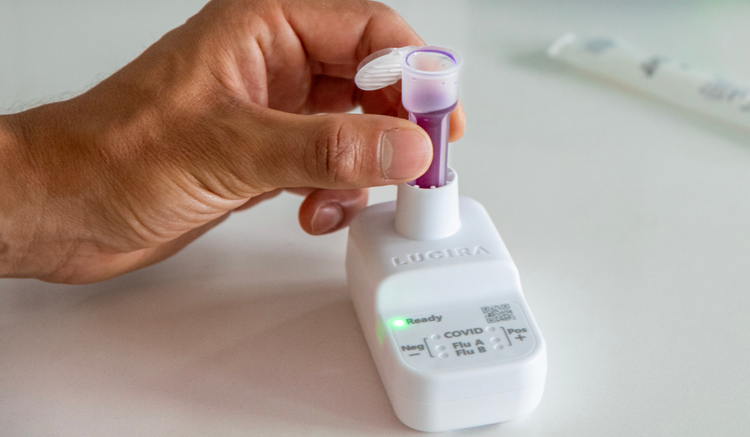
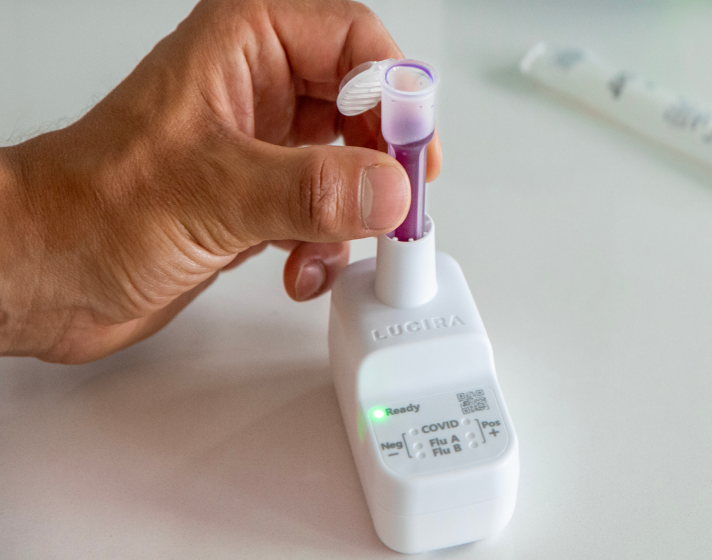
Easy.
Order LUCIRA® by Pfizer for your practice and offer single-use molecular testing without the need for equipment calibration, maintenance, capital investment, or extensive training.1

