

TECHNOLOGY
Molecular technology in your hands
See LUCIRA® by Pfizer COVID-19 & Flu Test in action
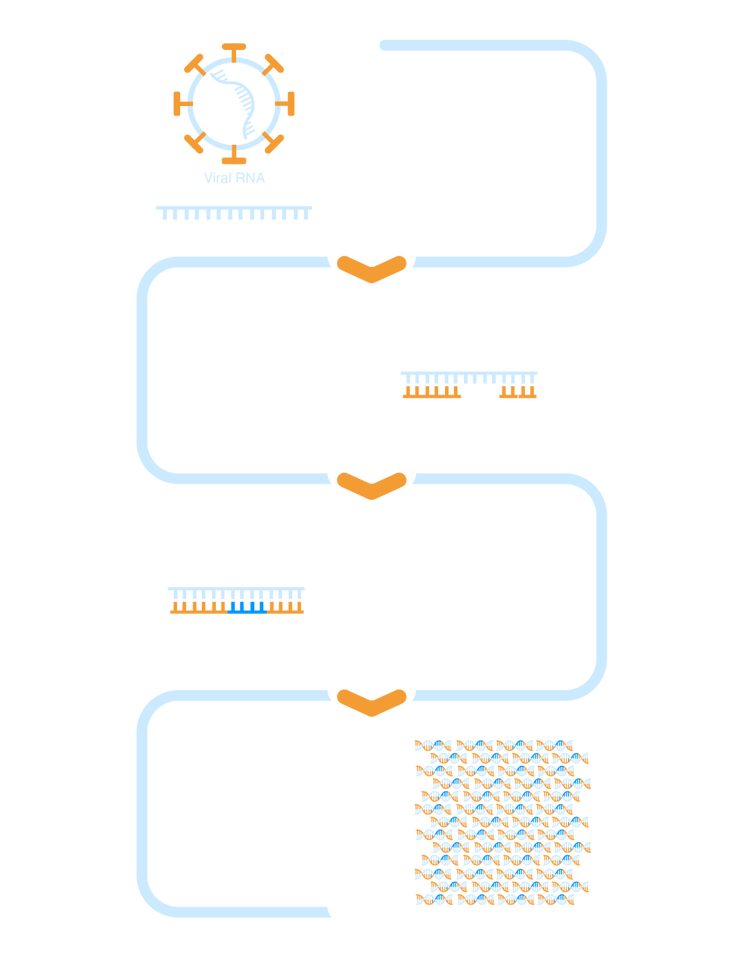
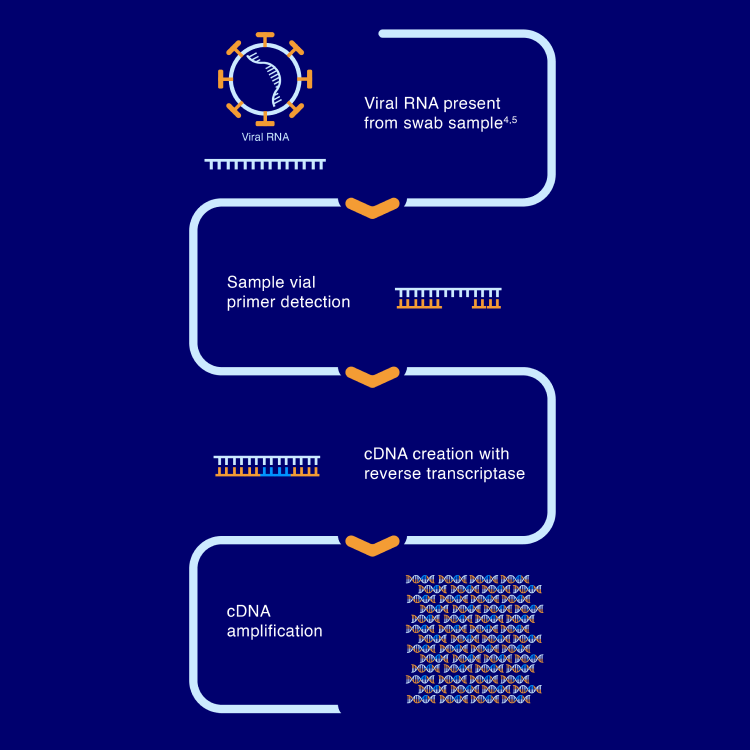
Harness the power of molecular amplification
LUCIRA® by Pfizer COVID-19 & Flu Test harnesses the efficient power of RT-LAMP.1
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) is a modern nucleic acid amplification technology that delivers the accuracy of polymerase chain reaction (PCR)* without thermocycling.2,3
LUCIRA® by Pfizer performs nucleic acid amplification at one consistent temperature, enabling testing to be performed on a palm-sized unit powered by 2 AA batteries.1,4
RT-LAMP detects pathogen-specific, target viral RNA and creates cDNA via reverse transcription, then amplifies the resulting cDNA millions of times. The amplification process changes the pH of the reaction, resulting in a colorimetric change and subsequent detection of the target organism.1,3
*The LUCIRA® by Pfizer COVID-19 & Flu Test provides a level of accuracy comparable to highly sensitive lab-based PCR tests. Negative results do not preclude SARS-CoV-2, influenza A, and/or influenza B infection and should not be used as the sole basis for patient management decisions.1
cDNA, complementary deoxyribonucleic acid; RNA, ribonucleic acid.

Proven high-accuracy* COVID-19 and Flu detection in head-to-head performance studies1
The LUCIRA® by Pfizer COVID-19 & Flu Test performed comparably in a head-to-head clinical trial and a surrogate study compared to highly sensitive lab-based PCR tests.1,6
Surrogate Sample Study1
The Surrogate Sample Testing Study compared LUCIRA® by Pfizer COVID-19 & Flu Test performance to that of FDA-cleared or authorized comparator methods.
- Samples were collected in viral transport medium and used to prepare contrived specimens for testing
- A total of 425 samples were evaluated, and the comparator assays were performed as per the cleared or authorized Instructions for Use
*The LUCIRA® by Pfizer COVID-19 & Flu Test provides a level of accuracy comparable to highly sensitive lab-based PCR tests. Negative results do not preclude SARS-CoV-2, influenza A, and/or influenza B infection and should not be used as the sole basis for patient management decisions.
LUCIRA® by Pfizer COVID-19 & Flu Test
Surrogate Sample Testing Study Results
|
Positive Percent Agreement |
Negative Percent Agreement |
|
|
COVID-19 (Total=406) |
97.3% (n=107/110; 95% Cl: 92.3%-99.1%) |
99.7% (n=295/296; 95% Cl: 98.1%-99.9%) |
|
Influenza A (Total=408) |
98.4% (n=60/61; 95% Cl: 91.3%-99.7%) |
100% (n=347/347; 95% Cl: 98.9%-100%) |
|
Influenza B (Total=407) |
95.3% (n=41/43; 95% Cl: 84.5%-98.7%) |
99.7% (n=363/364; 95% Cl: 98.5%-100%) |
Prospective Sample Study1
The Prospective Sample Study evaluated clinical performance of the LUCIRA® by Pfizer COVID-19 & Flu Test at 7 US study sites during the 2022–2023 flu season. 1161 anterior nasal swab samples were collected from subjects with signs and symptoms consistent with respiratory infection.
- LUCIRA® by Pfizer performance was evaluated using samples collected by participants
- Comparator assay test (FDA EUA SARS-CoV-2 molecular assay and FDA-cleared Influenza A and B molecular assay) samples were collected by a healthcare professional as indicated in the IFUs
LUCIRA® by Pfizer COVID-19 & Flu Test
Prospective Study Results
|
Positive Percent Agreement |
Negative Percent Agreement |
|
|
COVID-19 (Total=952) |
88.3% (n=83/94; 95% Cl: 80.2%-93.3%) |
100% (n=858/858; 95% Cl: 99.6%-100%) |
|
Influenza A (Total=1066) |
90.1% (n=109/121; 95% Cl: 83.5%-94.2%) |
99.3% (n=938/945; 95% Cl: 98.5%-99.6%) |
|
Influenza B (Total=1065) |
N/A† (n=0/0) |
99.9% (n=1064/1065; 95% Cl: 99.5%-100%) |
†No samples positive for influenza B were collected during the study due to low levels of influenza B in circulation at the time.1
Surveillance of emerging strains of viruses1,6
Pfizer performs routine surveillance of emerging SARS-CoV-2 and Influenza strains and will continue to monitor the situation with emerging variants. This includes the SARS-CoV-2 strains known as Omicron (and its BA subvariants), Eris, and Pirola.
Technical briefs that list SARS-CoV-2 variants and influenza strains to which the LUCIRA® by Pfizer COVID-19 & Flu Test is reactive are available here.
CI, confidence interval; EUA, emergency use authorization; FDA, US Food & Drug Administration; IFU, instructions for use.